A1R/A2AR RECEPTOR ANTAGONISTS
Project Summary
CVXL-0069 Project aims to develop a new mixed adenosine A1R/A2AR receptor antagonist drug, a novel mechanism of action that acts at the level of the striatum (movement control) and the cortex (sleep-wake and memory) to:
- Treat non-motor symptoms – drowsiness and memory impairment – and motor symptoms of Parkinson’s disease,
- Limit the use of dopaminergic agonist therapy in early Parkinson’s patients,
- Treat wakefulness disorders, excessive daytime sleepiness by a non-dopaminergic approach, linked to the use of drugs that disrupt the sleep-wake cycle.
CPh-1008, the first candidate in the series, has demonstrated its efficacy in increasing the duration of action of levodopa in a recognized preclinical model of Parkinson’s disease. Other candidates with a stronger A1R component, such as CPh-2024, are currently being characterized.
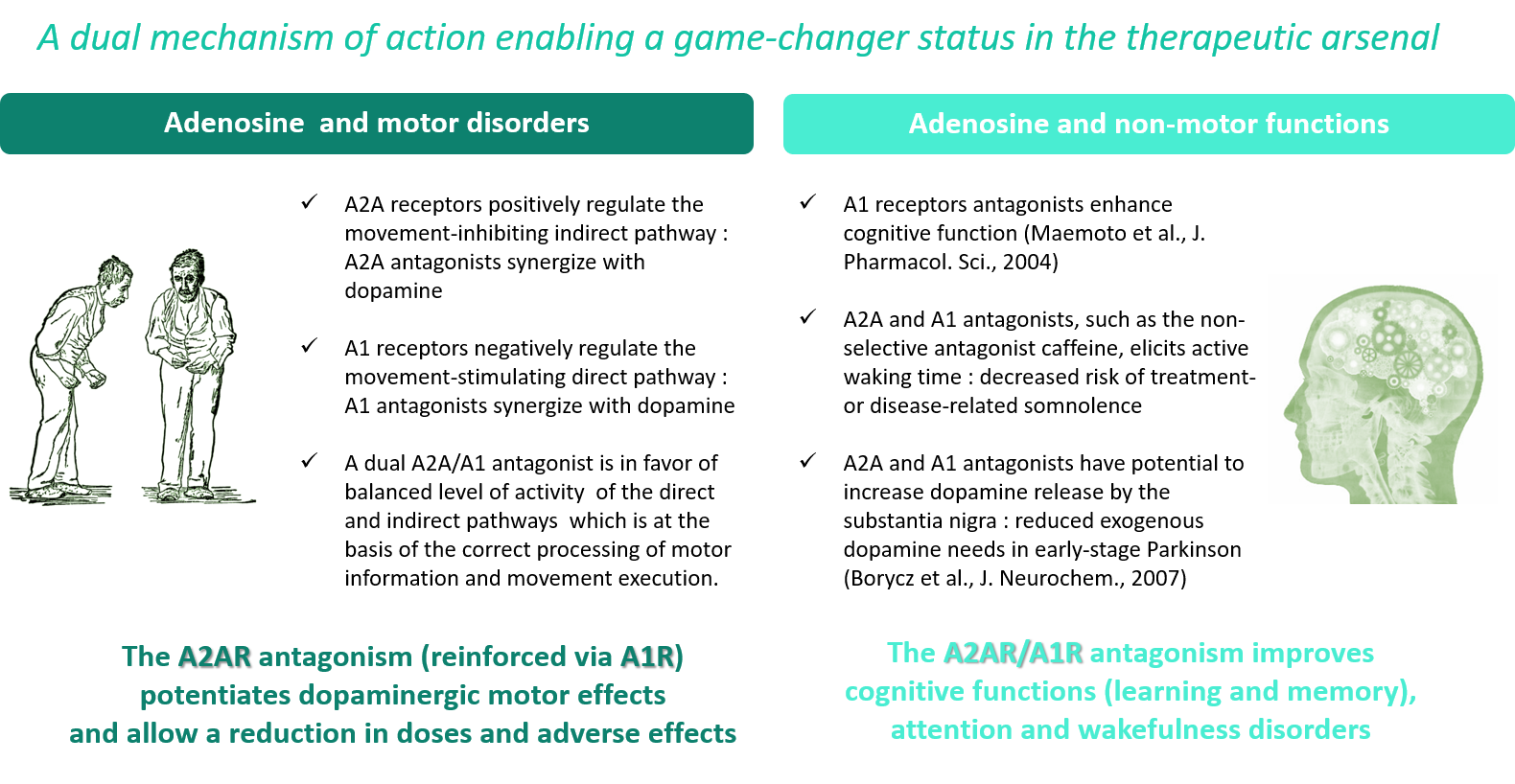
Therapeutic Background and Objectives
Adenosine is a neuromodulator involved in multiple brain activities. It acts through four receptor subtypes (AxR), including the A1R and A2AR receptors, which are particularly important in movement disorders such as Parkinson’s disease. Istradefylline (Nouriast®), a specific antagonist of the binding of adenosine to A2AR receptors, was recently approved by the FDA for this indication.
Non-selective adenosine receptor antagonists, acting on A2AR but also on A1R, such as caffeine, are well known to increase arousal and also have favorable effects on memory impairment, two early and disabling symptoms of Parkinson’s disease. While A2AR receptor blockade improves motor disturbances, it has little effect on non-motor symptoms and a drug that acts on both A1R and A2AR will allow it to act on both sides of the disease. Finally, inhibition of A2A and A1 receptors have the potential to increase the release of dopamine from the black substance and thus reduce the drug requirement for levodopa in early stage patients.
